-
Redox-active covalent organic frameworks for multivalent metal ion storage (RACOF-MMIS)
Project leadership
Prof. Dr. Eike Brunner
Technische Universität Dresden
Fakultät Chemie und Lebensmittelchemie
Professur für Bioanalytische ChemieProf. Dr. Xinliang Feng
Technische Universität Dresden
Fachrichtung Chemie und Lebensmittelchemie
Professur für Molekulare FunktionsmaterialienProf. Dr. Stefan Kaskel
Technische Universität Dresden
Fakultät Chemie und Lebensmittelchemie
Professur für Anorganische Chemie IProject description
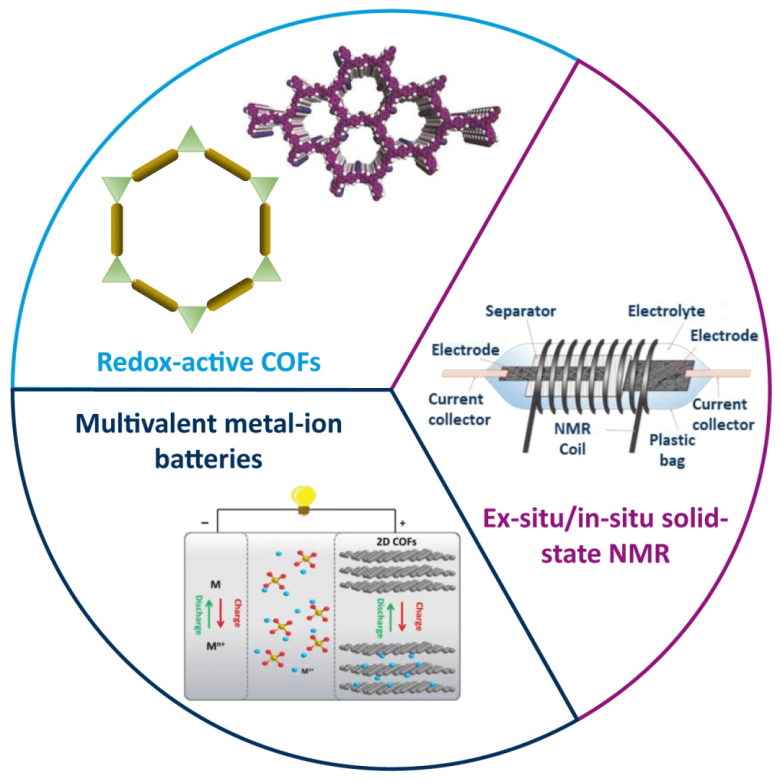
Owing to the natural abundance and reversible multielectron redox chemistries, multivalent metal-ion batteries (divalent: Mg2+, Zn2+, Ca2+, etc. trivalent: Al3+, etc.) have gained significant attention as the promising alternatives for post-Li-ion battery technologies. However, the big challenge lies in the limited availability of high-performance cathode materials with high capacity, good reversibility and long-term cyclability. In this regard, redox-active covalent organic frameworks (COFs) are of immense interest as host materials for multivalent metal ions, as COFs show large specific surface areas, tailorable pore structure and active sites, and unique coordination chemistry with multivalent metal ions.
This project plans to design and synthesize new redox-active COFs, explore their applications as cathode materials for multivalent metal-ion storage (Zn2+, Mg2+, and Al3+ in the first stage of SPP project), and try to uncover the multivalent metal-ion storage mechanism with the assistance of in-situ/ex-situ solid-state nuclear magnetic resonance characterization technology.
Ex-situ solid-state NMR experiments on electrolyte-loaded samples will deliver information about ion intercalation in the electrode materials. Interaction sites and the coordination state of NMR-active ions like 27Al will be determined. In-situ NMR experiments will provide information on electrode material - cation interactions under applied voltage. The materials will also be studied by solid-state NMR after cycling in order to evaluate possible aging and degradation processes at the molecular level.
The success of this project will provide insightful knowledge about the design and synthesis of favorable COFs with rich redox active centers (like imide, carbonyl, hexaazatrinaphthalene, sulfidic, polysulfide), chemically stable linkages (such as sp2-carbon (C=C), imide (C(=O)-N) and triazine (cyclic –C=N)), and precisely defined porous structures. Through the electrochemical research and mechanistic studies, the project will offer significant understanding underlying the multivalent metal-ion storage chemistries and establish the structure-performance relationship, which will guide the future development of novel high-performance COF electrodes for multivalent metal-ion batteries.
Grafik
Graphic: Prof. Dr. Eike Brunner, TU Dresden / Prof. Dr. Xinliang Feng, TU Dresden / Prof. Dr. Stefan Kaskel, TU Dresden -
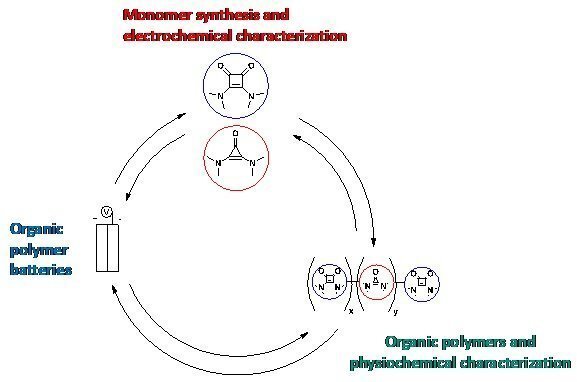
Polyimidazoles as Redox-active Electrodes for Deep Eutectic Solvent Electrolytes in Polymer Batteries
Project leadership
Prof. Dr. Timo Jacob
Universität Ulm
Fakultät für Naturwissenschaften
Institut für ElektrochemieProf. Dr. Alexander Kühne
Universität Ulm
Fakultät für Naturwissenschaften
Institut für Organische Chemie IIIProject description
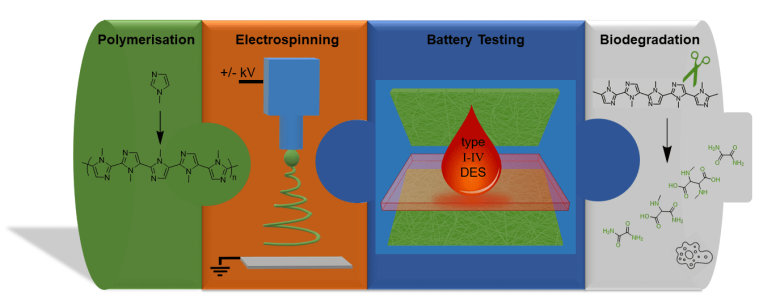
The aim of the project is to develop a fully biodegradable polymer battery. For this purpose, polyimidazole is used as a redox-active electrode material and combination with deep eutectic solvents as electrolytes to form a battery. Polyimidazole is both easily oxidized and reduced and therefore represents an excellent material for both cathode and anode. Imidazole monomers can be derived from renewable resources, while polyimidazole is biodegradable via oxidation by composting bacteria. Electrospinning is used to produce a non-woven fiber-electrode with very high surface area from the polymer. Metal-free deep eutectic solvents (DES) will be used as electrolytes that are accessible from biologically-derived and non-cytotoxic compounds.
In this project, we will optimize the imidazole polymers for superior performance in charge storage devices. Furthermore, suitable DES will be screened for their electrochemical properties and tested together with the polyimidazole electrodes in battery cells. Finally, we will investigate the biocompatibility and degradability of our material systems.
Grafik
Graphic: Prof. Dr. Timo Jacob, Uni Ulm / Prof. Dr. Alexander Kühne, Uni Ulm -
Electrochemical Construction of Functional Conductive Polymers as Free-Standing Cathode for Li-S Batteries: Synthesis, Operando Analysis, and Simulation
Project leadership
Prof. Dr. Joachim DzubiellaExternal link
Albert-Ludwigs-Universität Freiburg
Physikalisches Institut
Angewandte Theoretische Physik - Computergestützte PhysikProf. Dr. Yan Lu
Universität Potsdam
Institut für ChemieDr. Sebastian RisseExternal link
Helmholtz-Zentrum Berlin für Materialien und Energie
Institut Weiche Materie und Funktionale Materialien
Lise-Meitner-CampusProject description
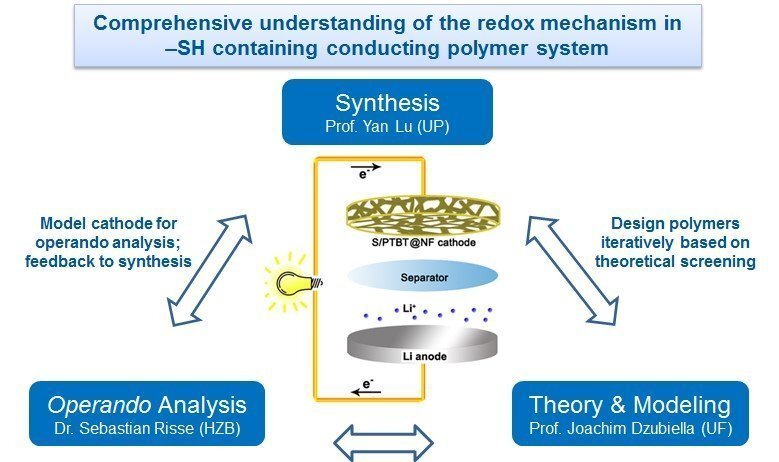
In this project, we propose the electrochemical construction, operando characterization as well as theoretical modeling of a conductive, flexible and free-standing cathode with the polymer poly(4-(thiophen-3-yl) benzenethiol) (PTBT) as sulfur host material for lithium sulfur (Li-S) batteries. In particular, PTBT can be deposited on the surface of nickel foam by an electropolymerization method, which enables the application of a highly porous and binder-free cathode for Li-S batteries. Sulfur can be copolymerized with a PTBT film through inverse vulcanization to form highly crosslinked copolymer P(S-PTBT), in which the feed sulfur is chemically bonded to the thiol groups of PTBT. By applying a novel in-house developed operando setup that can perform more than three different measurements simultaneously while the cell is in operation, we plan to gain mechanistic knowledge about important reaction steps, e.g., the process that chemically bonds the sulfur to the polymer backbone during electrochemical oxidation. In particular, UV/vis-, Raman-, and impedance-spectroscopy in combination with either X-ray imaging or small angle scattering will be used.
The experiments will be tightly linked to extensive modeling and simulations efforts on electronic and molecular scales: We will study the stability and the electronic/molecular structure of the sulfur covalently bonded to the thiol-containing neutral and charged polymers using state-of-the-art exchange-correlation functionals in density functional theory (DFT). In order to elucidate the structure-performance relationship of the proposed cathodes, we will compare operando analysis such as UV/vis spectroscopy directly with results from DFT calculations and DFT-optimized, classical molecular dynamics (MD) simulations.
Hence, by the combination of synthesis, operando analysis and simulation, we aim to gain a better mechanistic understanding of the energy storage processes and structure-property-function relationships in this system. This knowledge will be used for a continuous improvement of material parameters that enhance the electrochemical performance next-generation Li-S batteries.
Grafik
Graphic: Prof. Dr. Joachim Dzubiella, Uni Freiburg / Prof. Dr. Yan Lu,, Uni Potsdam / Dr. Sebastian Risse, HZB -
Aluminum- and magnesium-organic polymer-based batteries (AMPERE)
Project leadership
Prof. Dr. Birgit Esser
Ulm University
Institute for Organic Chemistry II and Advanced MaterialsProf. Dr. Ingo Krossing
Albert-Ludwigs-Universität Freiburg
Institut für Anorganische und Analytische ChemieProject description
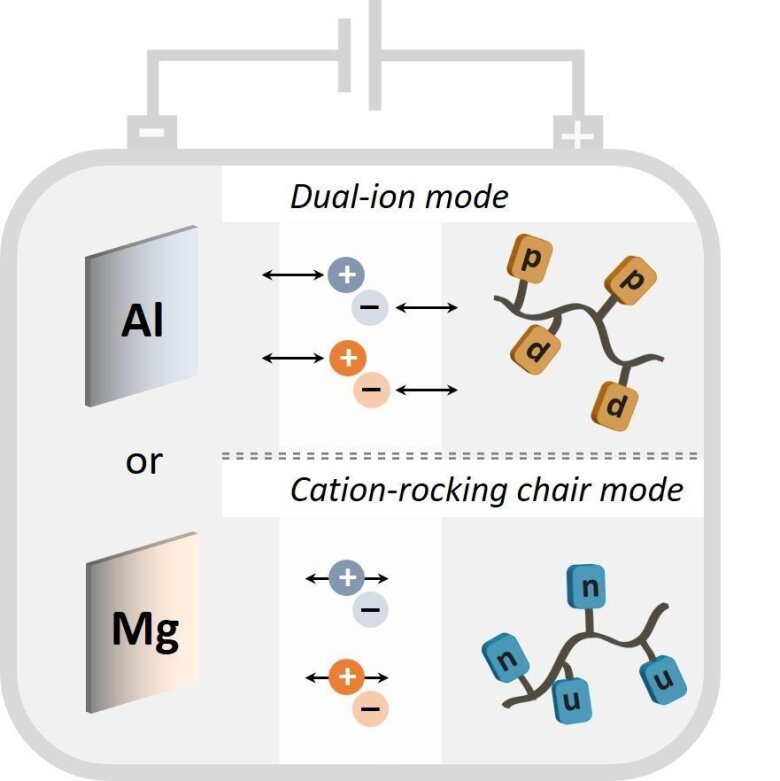
The goal of the proposed project is to develop aluminum- and magnesium-organic polymer-based batteries. Both p- and n-type redox-active polymers with phenothiazines or quinone-containing molecules as redox-active units will be synthesized as cathode-active materials. Aluminum- and magnesium-based electrolytes will be developed, which are compatible with the organic redox polymers and allow for reversible plating of Al/Mg on the anode. The redox polymers will be tested in full cells using aluminum and magnesium as anode, either operating in dual-ion mode for p-type polymers or cation rocking chair-mode for n-type redox polymers. Mechanistic experimental and computational investigations will allow to gain understanding on the ion insertion processes in the polymer-based electrodes.
Grafik
Graphic: Prof. Dr. Birgit Esser, Uni Freiburg / Prof. Dr. Ingo Krossing, Uni Freiburg -
Development of novel redox-active polymers based on benzimidazole, benzoxazole and benzothiazole – a combined theoretical and experimental screening approach
Project leadership
Prof. Dr. Doreen Mollenhauer
Justus-Liebig-Universität Gießen
Fachbereich Biologie und Chemie
Physikalisch-Chemisches InstitutProf. Dr. Ulrich S. Schubert de
Friedrich-Schiller-Universität Jena
Institut für Organische Chemie und Makromolekulare ChemieProject description
Polymer-based batteries have gained much research interest in recent years due to their interesting properties. Their virtues include their light-weight, the possibility to renounce critical metals, the utilization of available elements and their better sustainability in fabrication and recycling. Different redox-active polymers have been investigated in the last years resulting in many structural motifs identified as potential electrode materials. Currently, there are still limited anode materials available.
Within this context, this joint project of the FSU Jena and the JLU Giessen will develop new redox-active polymers based on three structural motifs – benzimidazoles, benzoxazoles and benzothiazoles – which are all pyridyl-substituted. The resulting (electrochemical) properties can be tuned by the substituents and the heteroatom in the five-membered ring (-NH-, NR-, -O-, -S-). A combined theoretical (JLU) and experimental (FSU) screening approach will be utilized to identify the most promising active materials. Firstly, suitable redox moieties will be investigated by the calculation and theoretical screening of different model compounds by DFT. Moreover, redox moieties with promising properties identified by the theoretical approach will be synthesized and their electrochemical properties will be studied. Based on this first screening suitable moieties for the integration into polymers will be selected. The second step of the project is the modelling of the polymers as well as their synthesis and electrochemical properties. This in turn will provide the polymeric materials with the best properties, which will be used for the fabrication of electrodes. These electrodes will be tested in (half)-cell tests.
Grafik
Graphic: Prof. Dr. Doreen Mollenhauer, Uni Gießen / Prof. Dr. Ulrich S. Schubert, FSU Jena -
Unraveling Degradation Mechanisms in Polymer-Based Dual-Ion Batteries and Development of Countermeasures for Performance Optimization
Project leadership
Dr. Tobias Placke
Westfälische Wilhelms-Universität Münster
MEET - Münster Electrochemical Energy TechnologyProf. Dr. Ulrich S. Schubert de
Friedrich-Schiller-Universität Jena
Institut für Organische Chemie und Makromolekulare ChemieProject description
Polymer-based batteries are considered as future candidates for sustainable energy storage, motivated by low-energy consuming processes during production of polymers, easy recyclability of the battery cells as well as the utilization of more abundant materials and replacement of critical metals. However, up to date, polymer-based batteries still suffer from various challenges in terms of their electrochemical performance, including poor energy density or cycling stability. In particular, there is still a significant lack of fundamental understanding of the capacity fading mechanisms and aging mechanisms at the electrode/electrolyte interfaces, i.e., of the three-dimensional “interphases” formed at the positive and negative electrodes.
In this project, a special type of polymer-based battery will be systematically investigated, i.e., a so-called polymer-based dual-ion battery (DIB) using n- and p-type organic materials for simultaneous storage of cations and anions, respectively. The DIB system differs from the classical cation or anion rocking-chair-type polymer batteries, as both charge carriers, cations and anions, are involved in the dual-ion storage mechanism. This offers various advantages such as a high versatility of possible cation-anion combinations as well as typically a high cell voltage, which might be achieved by suitable host polymer materials. Different strategies will be pursued within this project in order to develop polymer-based DIB systems having an improved energy density and stability. These approaches include
- the design of new polymeric materials featuring a higher positive electrode potential (“voltage tuning”),
- the development of hybrid systems such as graphite || polymer systems offering a high cell voltage,
- all-polymer DIB systems, focusing on different concepts including ambipolar polymers and a “reverse all-polymer DIB system”.
The different polymer DIB systems will be comprehensively studied in terms of their electrochemical performance, with particular focus on the impact of the electrolyte and interphases on the reversible capacity and stability during prolonged charge/discharge cycling. For this purpose, the electrolyte will be specifically designed for each polymer system following different strategies such as the use of highly concentrated electrolyte or the addition of electrolyte additives for interphase design and stability enhancement. Furthermore, different ex-situ and in-situ analyses will be applied to gain sig¬nificant and comprehensive insights into mechanistic properties of cation/anion storage, stability of the polymer materials and the role of the interphase formation.It is expected that the fundamental insights gained in this project will be of high importance for the development of improved polymer active materials and optimized electrolytes for polymer-based DIB cells with high energy density and cycling stability.
Grafik
Graphic: Dr. Tobias Placke, WWU Münster / Prof. Dr. Ulrich S. Schubert, FSU Jena -
Development of Polymer Electrolytes Complementary to Model Active Systems for Polymer-based Batteries
Project leadership
Prof. Dr. Ulrich S. Schubert de
Friedrich-Schiller-Universität Jena
Institut für Organische Chemie und Makromolekulare ChemiePD Dr. Petra Uhlmann
Leibniz-Institut für Polymerforschung Dresden e.V. (IPF)Project description
This collaborative research project of IPF Dresden and FSU Jena aims at the development of novel polymer electrolytes complementary to model active materials for polymer-based bat-teries. The electrolytes will be developed with tailor-made properties regarding ion transport, mor¬phology, thermal and electrochemical stability as well as compatibility to the electrodes (i.e. active material and conductive additive). The principle transport mechanisms will be studied, just as the influence of the electrolyte structure and interfaces on the cell performance. The main scientific aim of the project is to gain a better understanding of correlations between the chemical and morphological structure of the cell components with the performance of model polymer batteries. For this, in a first approach, polymeric single-ion conductors for anions will be synthesized, which are suitable electrolyte systems for active polymeric materials requiring anion-shuttling. The second approach aims at a charge transport reversal by equipping the active materials with charged species. These research activities will extended by developing triblock copolymer containing all necessary components for a molecular battery.
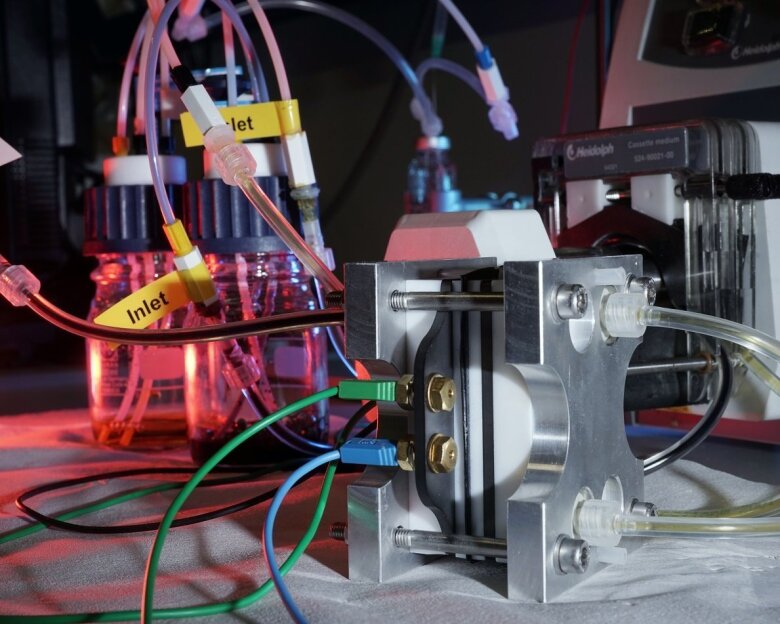
Redox-Flow-Batterie im Labor
Image: Jan-Peter Kasper (University of Jena) -
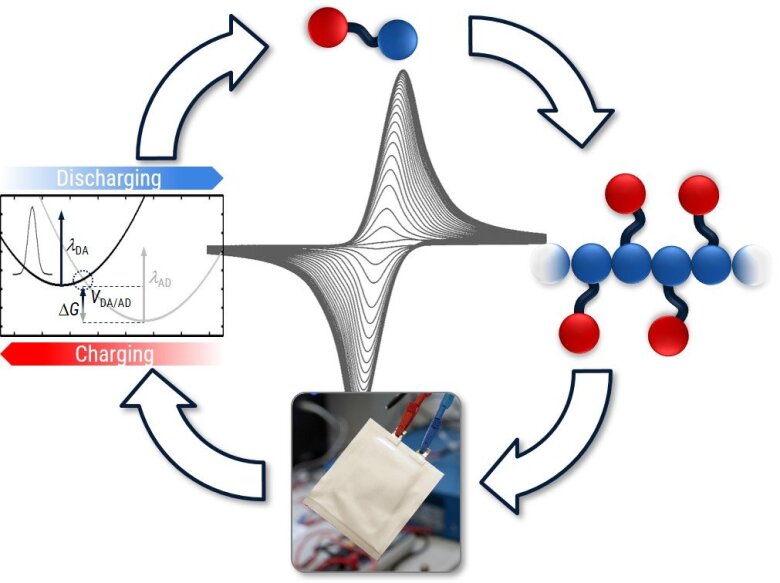
Redox-active organic subunits in linear amorphous polymers versus highly ordered COFs as hierarchical structured battery electrode materials – PROMISE
Project leadership
Dr. Oliver Dumele
Humboldt-Universität zu Berlin
Institut für Chemie
Arbeitsgruppe Organische Chemie und funktionale MaterialienProf. Dr. Birgit Esser
Ulm University
Institute for Organic Chemistry II and Advanced MaterialsProject description
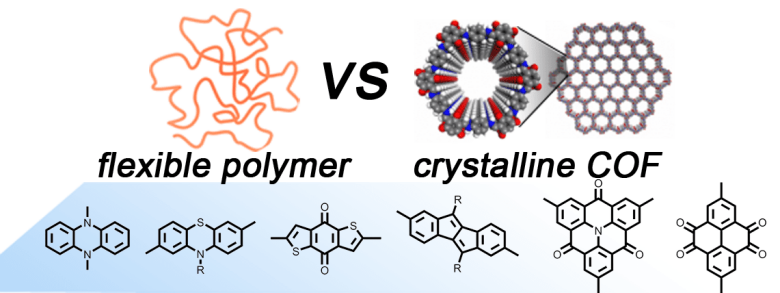
A large variety of redox-active organic small molecules and polymers has been studied as potential electrode materials for organic batteries. However, little is known on the relative alignment of the redox-active subunits in polymeric materials. Non-covalent interactions as well as the effect of crystalline alignment and hierarchical structures of multicomponent systems can have a significant impact on the battery performance.
We systematically study the comparison of such redox-active monomeric units in linear, amorphous polymers versus crystalline two-dimensional covalent organic frameworks (2D-COFs). The synthesis of novel 2D-COFs and their corresponding linear polymers bearing directly comparable redox-active units will be achieved within this SPP. Fundamental mechanisms of charge and radical stabilization will be elucidated with spectroscopic and electrochemical methods, and battery cell fabrication using the linear polymers and 2D-COFs as electrode-active materials will allow for a structure–activity relationship study, where the final performance of the devices are evaluated and tuned.
Grafik
Image: Dr. Oliver Dumele, HU Berlin / Prof. Dr. Birgit Esser, Uni Freiburg -
Polymers based on squaric acid amides and cyclopropenium cations as new electrode materials for energy storage: Synthesis and electrochemical characterization
Project leadership
Dr. Dominic Bresser
Karlsruher Institut für Technologie (KIT)
Helmholtz-Institut Ulm für Elektrochemische Energiespeicherung (HIU)Professor Dr. Max Martin Hansmann
Technische Universität Dortmund
Fakultät Chemie und Chemische Biologie
Organische ChemieProfessor Dr. Patrick Théato
Karlsruher Institut für Technologie (KIT)
Institut für Technische Chemie und PolymerchemieProject description
This project aims at combing synthetic and electrochemical expertise to drive the development of new small electro-active organic molecules and their incorporation within polymers. The most investigated organic radical battery systems are based on TEMPO containing polymers. Yet, to improve their performance and in particular their energy density, new types of smaller organic redox active molecules are urgently needed. Herein, we focus on the smallest known redox active organic species, namely derivatives of cyclopropenium cations and squaric acid amides, as building blocks for a new class of redox active polymers suitable as materials for electrochemical energy storage. Such polymeric active materials in organic radical batteries shall fulfill a number of criteria:
- stable and reversible redox states,
- easy synthetic access, and
- large positive and/or negative redox potentials in order to obtain high full-cell voltages.
Grafik
Graphic: Dr. Dominic Bresser, KIT / Prof. Dr. Max Martin Hansmann, TU Dortmund / Prof. Dr. Patrick Théato, KIT -
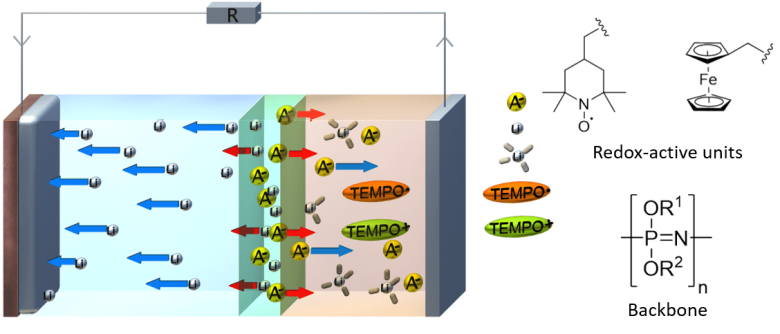
Insight into doping mechanisms of polymer electrolyte / redox-active organic radical polymer lamellar composites
Project leadership
PD Dr. Gunther BrunklausExternal link
Forschungszentrum Jülich
Helmholtz-Institut MünsterProf. Dr. Rüdiger-A. EichelExternal link
Forschungszentrum Jülich GmbH
Institut für Energie- und Klimaforschung (IEK)
Grundlagen der Elektrochemie (IEK-9)Prof. Dr. Andreas HeuerExternal link
Westfälische Wilhelms-Universität Münster
Institut für Physikalische ChemieProject description
In this project, multilayer polymer batteries are investigated in a holistic approach consisting of synthesis, electrochemical characterization, theoretical and numerical modeling as well as a broad range of EPR techniques. The lamellar cells consist of a lithium metal anode, a lithium ion conducting polymer electrolyte layer, a polymeric anion and cation reservoir as well as an anion conducting redox-active polymer as cathode. The properties of this cell design are compared in detail with those of PolyTEMPO, a well-established redox polymer system for liquid electrolytes.
The synthesis part includes production and processing of the polymer materials to lamellar composites as well as their comprehensive electrochemical characterization. The cathode material is optimized via chemical functionalization, especially with respect to the cell voltage, charge and discharge kinetics as well as the anion mobility within the cathode, which is important for charge neutrality and charge transfer.
Details of the radical transfer mechanisms and the occurring ionic species are elucidated using continuous-wave and, if possible, pulsed EPR methods. Spectral characteristics of pristine and cycled materials (post mortem) are compared, including characterization of typical distances and the distribution of radical species (PELDOR, DEER, ENDOR, HYSCORE). In-operando EPR will be performed on selected samples in order to follow the temporal evolution of radical species by their fingerprint signal and to reveal molecular details of charge transfer processes.
Additional insights into mechanistic details of electron and ion transport within the (polymer) layered composite are gained by theoretical and numerical modeling. Quantum chemical methods are used to characterize electronic properties of the redox-active polymers, while the long-range ion transport and possible doping mechanisms of organic cathodes are analyzed by molecular dynamic (MD) simulations. In addition, the MD simulations will be augmented by Monte Carlo steps to model electron transfer processes across several monomers, which will be rationalized in comparison with experimental data.
Overall, the systematic approach is intended to derive a fundamental understanding of relevant electrochemical processes, from elementary electron transfer to ion transport through interfaces. These findings will serve as a guideline for the identification of promising redox-active polymer materials and the targeted design of interfaces within the (polymer) multilayer structures to promote the future development of efficient organic radical batteries that contain as little solvent as possible or are even entirely solvent-free. This includes the alternative organic electrode materials developed within this project.
Grafik
Graphic: Dr. Gunther Brunklaus, Forschungszentrum Jülich / Prof. Dr. Rüdiger-A. Eichel, Forschungszentrum Jülich GmbH, Prof. Dr. Andreas Heuer, WWU Münster -
Development of active materials for organic batteries based on electropolymerized polymers equipped with stable organic radicals
Project leadership
Dr. Christian Friebe
Friedrich-Schiller-Universität Jena
Institut für Organische Chemie und Makromolekulare ChemieDr. Stephan Kupfer
Friedrich-Schiller-Universität Jena
Chemisch-Geowissenschaftliche Fakultät
Institut für Physikalische Chemie
Arbeitsgruppe Theoretische ChemieProject description
Today's battery technology is mostly based on metals, such as lithium, lead, cobalt, or nickel. However, their limited natural occurrence as well as their toxicity and the resulting disposal problems limit their usability. Alternatively, polymeric compounds can be used. In this context, polymers based on stable organic radicals were studied intensively revealing promising charge-storage properties, in particular a superior redox reaction kinetic.
However, such materials suffer from poor electrical conductivity, which limits the applicable charging/discharging rates, cancelling the advantageous electron-transfer characteristic. A promising approach to overcome this problem is the incorporation of conductive, i.e., conjugated, polymers. These materials offer several beneficial features that can be exploited for an organic battery:
- As semiconductors, they offer electronical conductivity;
- they can be applied via electropolymerization, offering an efficient way to prepare insoluble polymeric structures directly on an electrode surface; and
- they themselves offer charge-storage capability.
While the storage capability of conjugated polymers is often restricted due to a sloping charge / discharge voltage, the electronical conductivity as well as the electrochemical processability present highly advantageous features in combination with a stable redox moiety such as organic radicals. Nevertheless, up to now, only a few examples were presented in the literature.
Thus, the toolbox of organic battery materials shall be expanded within this project by combination of stable organic radicals with electropolymerizable moieties to gain improved electrochemical stability as well as electronical conductivity.
Grafik
Graphic: Dr. Christian Friebe, FSU Jena /Dr. Stephan Kupfer, FSU Jena -
Characterization of fabrication-microstructure-property relationships for polymer-based battery materials, combining tomographic 3D imaging with modeling and simulation
Project leadership
Prof. Dr. Thomas CarraroExternal link
Helmut-Schmidt-Universität
Universität der Bundeswehr Hamburg
Fakultät für Maschinenbau
Professur für Angewandte MathematikDr. Ingo MankeExternal link
Helmholtz-Zentrum Berlin für Materialien und EnergieProf. Dr. Volker SchmidtExternal link
Universität Ulm
Fakultät für Mathematik und Wirtschaftswissenschaften
Institut für StochastikProject description
The 3D morphology of battery electrodes is crucial for their performance and degradation behavior. However, in case of polymer batteries fabrication-microstructure-property relationships are not yet well understood.
In this project, (operando) characterization of the 3D structure and morphology of polymer-based battery electrodes at nano- and micrometer scale is used to obtain an accurate knowledge of the 3D morphology of experimentally manufactured electrodes and their functional properties. From the tomographic data sets, digital twins are created using stochastic models, which serve as a basis for the virtual design and testing of electrode materials. Then spatially resolved numerical simulations of charge transport together with a multiscale electrochemical model will be used to investigate how different electrode morphologies affect the electrochemical processes.
The project will help to pave the way for the fabrication of appropriate and stable hierarchical 3D morphologies on the micro- and nanometer scale that ensure simultaneous ionic and electronic conductivity through the electrode, leading to improved electrochemical performance of polymer-based batteries.
Links: FIB/SEM-Tomographie einer Polymer-Verbundelektrode (Probe des Kompositmaterials - PTMA, Super P, PVdF - zur Verfügung gestellt von der Schubert-Gruppe). Rechts: Interaktionsdiagramm für das Teilprojekt Carraro/Manke/Schmidt.
Graphic: Dr. Thomas Carraro, Helmut-Schmidt-Universität / Dr. Ingo Manke, HZB / Prof. Dr. Volker Schmidt (Uni Ulm) -
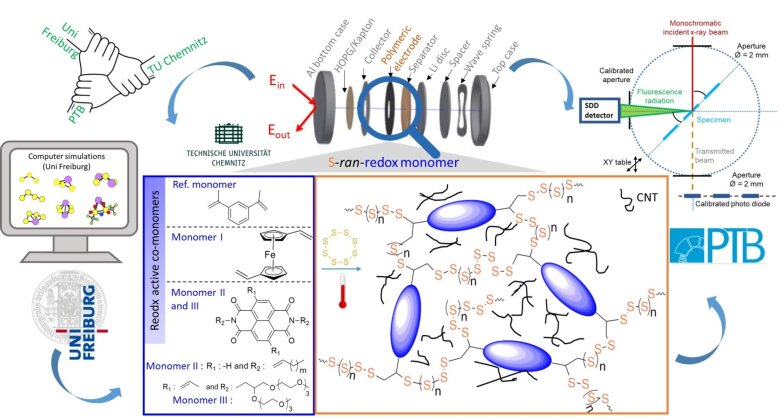
Novel Li-S batteries with dual redox couples: design, synthesis, in operando X-ray studies and theoretical modelling
Project leadership
Dr. Matthias Müller
Physikalisch-Technische Bundesanstalt (PTB)
Abteilung 7 - Temperatur und SynchrotronstrahlungProf. Dr. Michael Sommer
Technische Universität Chemnitz
Fakultät für Naturwissenschaften
Institut für ChemiePD Dr. Michael Walter
Albert-Ludwigs-Universität Freiburg
FIT Freiburger Zentrum für interaktive Werkstoffe
und bioinspirierte TechnologienProject description
In this project, novel materials for LiS cathodes with additional organic redox couples will be designed, synthesized, characterized, theoretically modelled, and investigated by highly advanced operando X-ray spectroscopies.
Using inverse vulcanization of sulfur with redox active divinyl monomers of varying redox potential, sulfur/ comonomer networks will be produced in which additional charge can be stored on the redox active comonomer. After designing and fabricating sulfur/ comonomer networks as cathode materials, tailored gel polymer electrolytes will be used that remain stable under the extended voltage window. The synthesis, characterization of novel polymeric battery materials, electrode preparation, optimization of suitable electrolytes, and detailed electrochemistry will be performed at Chemnitz University of Technology.
Physikalisch-Technische Bundesanstalt will investigate the novel battery electrodes by in operando near edge X-ray absorption fine structure (NEXAFS) spectroscopy, X-ray emission spectroscopy and reference-free X-ray fluorescence analysis. These X-ray experiments will reveal both the mutual influence of the two redox couples (sulfur and redox active cross-linker) as well as mechanistic details and side reactions during battery operation.
These investigations will be further supported by DFT simulations from the University of Freiburg to understand the stability and the electronic structure of the various species involved. Moreover, the theoretical part covers the prediction of NEXAFS spectra on an absolute scale to enable reliable identification of species. The simulations will further stimulate optimization of network materials.
The synergies from this joint theoretical and experimental project will contribute to enhanced mechanistic understanding as well as more stable, and more efficient, materials for both Li-S and polymer batteries.
Grafik
Graphic: Dr. Matthias Müller,PTB / Prof. Dr. Michael Sommer, TU Chemnitz / PD Dr. Michael Walter, Uni Freiburg